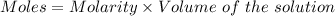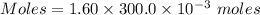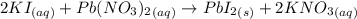You mix 285.0 ml of 1.20 m lead(ii) nitrate with 300.0 ml of 1.60 m potassium iodide. the lead(ii) iodide is insoluble. which of the following is false?
select one:
a. the final concentration of pb2+ ions is 0.174 m.
b. you form 111 g of lead(ii) iodide.
c. the final concentration of k+ is 0.821 m.
d. the final concentration of no3– is 0.821 m.
e. all are true.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
You mix 285.0 ml of 1.20 m lead(ii) nitrate with 300.0 ml of 1.60 m potassium iodide. the lead(ii) i...
Questions






Advanced Placement (AP), 14.09.2021 22:00

Chemistry, 14.09.2021 22:10

Mathematics, 14.09.2021 22:10

Mathematics, 14.09.2021 22:10


History, 14.09.2021 22:10




Mathematics, 14.09.2021 22:10




Mathematics, 14.09.2021 22:10

English, 14.09.2021 22:10