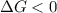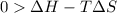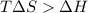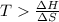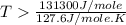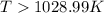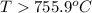Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
For the reaction c(s)+h2o(g)→co2(g)+h2(g) δh∘=131.3kj/mol and δs∘=127.6j/k⋅mol at 298k. at temperatu...
Questions

Mathematics, 05.02.2021 18:50

Health, 05.02.2021 18:50

History, 05.02.2021 18:50

Mathematics, 05.02.2021 18:50



Mathematics, 05.02.2021 18:50




Mathematics, 05.02.2021 18:50

English, 05.02.2021 18:50




Mathematics, 05.02.2021 18:50




Physics, 05.02.2021 18:50

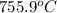 this reaction is spontaneous under standard conditions.
this reaction is spontaneous under standard conditions.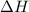 = 131.3 KJ/mole = 131300 J/mole
= 131.3 KJ/mole = 131300 J/mole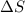 = 127.6 J/mole.K
= 127.6 J/mole.K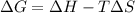
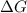 is negative or we can say that the value of
is negative or we can say that the value of