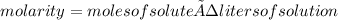Chemistry, 09.12.2019 18:31 jonmorton159
Urea, (nh2)2co, is a product of metabolism of proteins. an aqueous solution is 37.2% urea by mass and has a density of 1.032 g/ml. calculate the molarity of urea in this solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

You know the right answer?
Urea, (nh2)2co, is a product of metabolism of proteins. an aqueous solution is 37.2% urea by mass an...
Questions




Mathematics, 12.01.2021 01:50



Mathematics, 12.01.2021 01:50

Mathematics, 12.01.2021 01:50

Mathematics, 12.01.2021 01:50


Biology, 12.01.2021 01:50


History, 12.01.2021 01:50


Arts, 12.01.2021 01:50

Mathematics, 12.01.2021 01:50



Mathematics, 12.01.2021 01:50