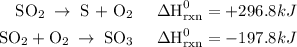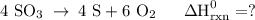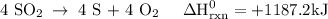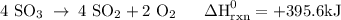Chemistry, 09.12.2019 18:31 ahhhhhhhh5509
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: 4 so3(g) → 4 s(s) + 6 o2(g) δh°rxn = ? given: so2(g) → s(s) + o2(g) δh°rxn = +296.8 kj 2 so2(g) + o2(g) → 2 so3(g) δh°rxn = -197.8 kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: 4...
Questions




Biology, 09.12.2020 20:30



History, 09.12.2020 20:30



Mathematics, 09.12.2020 20:30

Chemistry, 09.12.2020 20:30



English, 09.12.2020 20:30


Mathematics, 09.12.2020 20:30


Computers and Technology, 09.12.2020 20:30

Mathematics, 09.12.2020 20:30

Mathematics, 09.12.2020 20:30

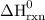 = 1582.8 kJ.
= 1582.8 kJ.