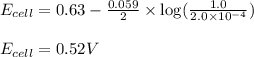Chemistry, 09.12.2019 19:31 LikeIke9418
The standard cell potential (e°cell) for the reaction below is +0.63 v. the cell potential for this reaction is when the concentration of zn2+ = 1.0 m and theconcentration of pb2+ = 2.0 x 10-4 m. pb2+(aq) + zn(s) > zn2+(aq) + pb(s) include details of how to calculate the need the steps. in advance.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3
You know the right answer?
The standard cell potential (e°cell) for the reaction below is +0.63 v. the cell potential for this...
Questions





Computers and Technology, 03.12.2020 22:20


Chemistry, 03.12.2020 22:20

Mathematics, 03.12.2020 22:20


Physics, 03.12.2020 22:20

Biology, 03.12.2020 22:20




Mathematics, 03.12.2020 22:20



English, 03.12.2020 22:20

Mathematics, 03.12.2020 22:20

Business, 03.12.2020 22:20

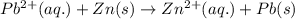
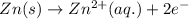
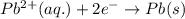
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Zn^{2+}]}{[Pb^{2+}]}](/tpl/images/0410/3244/a3f7c.png)
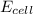 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V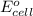 = standard electrode potential of the cell = 0.63 V
= standard electrode potential of the cell = 0.63 V![[Zn^{2+}]=1.0M](/tpl/images/0410/3244/26443.png)
![[Pb^{2+}]=2.0\times 10^{-4}M](/tpl/images/0410/3244/3bdd9.png)