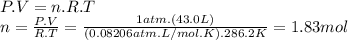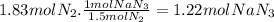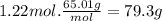The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of sodium azide, which produces large volumes of nitrogen gas. 1. write a balanced chemical equation, including physical state symbols, for the decomposition of solid sodium azide ( nan3 ) into solid sodium and gaseous dinitrogen. 2. suppose 43.0l of dinitrogen gas are produced by this reaction, at a temperature of 13.0°c and pressure of exactly 1atm . calculate the mass of sodium azide that must have reacted. round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1


You know the right answer?
The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of...
Questions

Social Studies, 26.11.2020 19:20




Mathematics, 26.11.2020 19:20


Social Studies, 26.11.2020 19:20




History, 26.11.2020 19:20

Biology, 26.11.2020 19:20

Computers and Technology, 26.11.2020 19:20

Social Studies, 26.11.2020 19:20


Geography, 26.11.2020 19:20


Chemistry, 26.11.2020 19:20