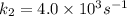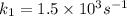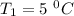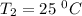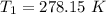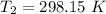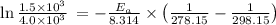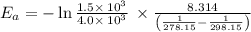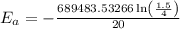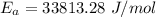Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Dinitrogen tetraoxide, n2o4, decomposes to nitrogen dioxide, no2, in a first-order process. if k = 1...
Questions

History, 23.07.2019 20:00

History, 23.07.2019 20:00



History, 23.07.2019 20:00




History, 23.07.2019 20:00

Mathematics, 23.07.2019 20:00



Geography, 23.07.2019 20:00


Biology, 23.07.2019 20:00



History, 23.07.2019 20:00


Mathematics, 23.07.2019 20:00

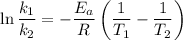
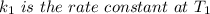
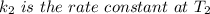
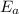 is the activation energy
is the activation energy