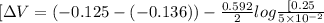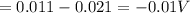Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
(a) compute the voltage at 25°c of an electrochemical cell consisting of pure lead immersed in a 5 ×...
Questions


Social Studies, 04.11.2020 02:50

Mathematics, 04.11.2020 02:50

Spanish, 04.11.2020 02:50

Advanced Placement (AP), 04.11.2020 02:50

Mathematics, 04.11.2020 02:50


Mathematics, 04.11.2020 02:50


Mathematics, 04.11.2020 02:50




Engineering, 04.11.2020 02:50

Mathematics, 04.11.2020 02:50


History, 04.11.2020 02:50


Geography, 04.11.2020 02:50

Mathematics, 04.11.2020 02:50

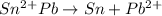
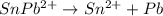
![[\Delta V=(V_{pb}^{0}-V_{sn}^{0})-\frac{0.592}{n}log\frac{[Sn^{2+}]}{[Pb^{2+}]}](/tpl/images/0410/3129/9e221.png)
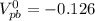
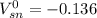
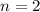
![[Sn^{2+}]= 0.25M](/tpl/images/0410/3129/b50c0.png)
![[Pb^{2+}]=5\times 10^{-2}M](/tpl/images/0410/3129/01669.png)