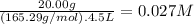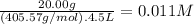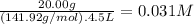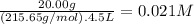Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3
You know the right answer?
What is the molarity of each ion present in aqueous solutions prepared by dissolving 20.00 g of the...
Questions

World Languages, 29.09.2019 19:30

Biology, 29.09.2019 19:30

Physics, 29.09.2019 19:30

Biology, 29.09.2019 19:30

Mathematics, 29.09.2019 19:30





Social Studies, 29.09.2019 19:30

Arts, 29.09.2019 19:30

History, 29.09.2019 19:30

English, 29.09.2019 19:30

Mathematics, 29.09.2019 19:30

Physics, 29.09.2019 19:30

Spanish, 29.09.2019 19:30

Mathematics, 29.09.2019 19:30