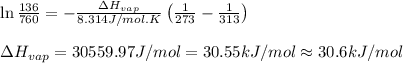Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

You know the right answer?
The vapor pressure of dichloromethane, ch2cl2, at 0 ∘c is 134 mmhg. the normal boiling point of dich...
Questions


Mathematics, 21.05.2021 08:40

Mathematics, 21.05.2021 08:40


Mathematics, 21.05.2021 08:40

Mathematics, 21.05.2021 08:40


Mathematics, 21.05.2021 08:40



Mathematics, 21.05.2021 08:40

Physics, 21.05.2021 08:40


Mathematics, 21.05.2021 08:40


Chemistry, 21.05.2021 08:40

Mathematics, 21.05.2021 08:40


Mathematics, 21.05.2021 08:40

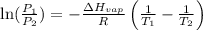
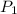 = vapor pressure at temperature
= vapor pressure at temperature 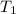 = 134 mmHg
= 134 mmHg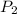 = vapor pressure at temperature
= vapor pressure at temperature 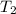 (atmospheric pressure) = 760 mmHg
(atmospheric pressure) = 760 mmHg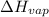 = molar heat of vaporization
= molar heat of vaporization![0^oC=[273+0]K=273K](/tpl/images/0410/2746/614a3.png)
![40^oC=[273+40]K=313K](/tpl/images/0410/2746/c9054.png)