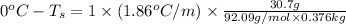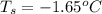Chemistry, 09.12.2019 19:31 nayelimoormann
Determine the freezing point depression of a solution that contains 30.7 g glycerin (c3h8o3, molar mass = 92.09 g/mol) in 376 ml of water. some possibly useful constants for water are kf = 1.86°c/m and kb = 0.512°c/m.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1
You know the right answer?
Determine the freezing point depression of a solution that contains 30.7 g glycerin (c3h8o3, molar m...
Questions

Computers and Technology, 23.04.2020 16:13



Biology, 23.04.2020 16:14




Chemistry, 23.04.2020 16:14


German, 23.04.2020 16:14

History, 23.04.2020 16:14


Biology, 23.04.2020 16:14






English, 23.04.2020 16:14

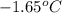
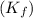 for water =
for water = 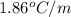

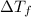 = change in freezing point
= change in freezing point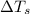 = freezing point of solution = ?
= freezing point of solution = ?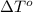 = freezing point of water =
= freezing point of water = 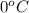
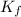 = freezing point constant for water =
= freezing point constant for water =