

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
Sodium carbonate can be made by heating sodium bicarbonate: 2nahco3(s) → na2co3(s) + co2(g) + h2o(g...
Questions


Geography, 21.07.2020 22:01

Mathematics, 21.07.2020 22:01

Mathematics, 21.07.2020 22:01

Biology, 21.07.2020 22:01

Mathematics, 21.07.2020 22:01

Spanish, 21.07.2020 22:01



English, 21.07.2020 22:01



Mathematics, 21.07.2020 22:01

French, 21.07.2020 22:01





Computers and Technology, 21.07.2020 22:01

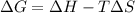
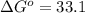
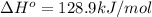
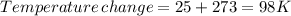
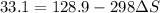
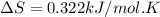
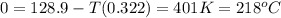
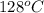 minimum temperature will the reaction become spontaneous.
minimum temperature will the reaction become spontaneous.

