
Chemistry, 09.12.2019 21:31 Knownothing
Fe(ii) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the solution, which converts fe(ii) to insoluble fe(iii):
4fe(oh)+(aq)+ 4oh-(aq)+o2(g)+2h2o(l) = 4fe(oh)3(s)
how many grams of o2 are consumed to precipitate all of the iron in 85ml of 0.075 m fe(ii).
explain step by step

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 23.06.2019 06:30
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2

Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1
You know the right answer?
Fe(ii) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the sol...
Questions

Biology, 23.07.2019 06:30


Mathematics, 23.07.2019 06:30


Mathematics, 23.07.2019 06:30

Mathematics, 23.07.2019 06:30



History, 23.07.2019 06:30

History, 23.07.2019 06:30



English, 23.07.2019 06:30



Health, 23.07.2019 06:30




Mathematics, 23.07.2019 06:30

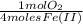 =
= 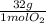 = 0,051g of O₂
= 0,051g of O₂

