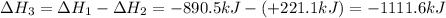Chemistry, 09.12.2019 22:31 santos200154
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 890.5 kj xco 3 ( s ) ⟶ xo ( s ) + co 2 ( g ) δ h = + 221.1 kj what is δ h for this reaction? x ( s ) + 1 2 o 2 ( g ) + co 2 ( g ) ⟶ xco 3 ( s )

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 890.5 kj xco 3 ( s ) ⟶ xo ( s ) + co...
Questions

Chemistry, 01.06.2021 09:30



Health, 01.06.2021 09:40



Mathematics, 01.06.2021 09:40

Physics, 01.06.2021 09:40


Mathematics, 01.06.2021 09:40


Engineering, 01.06.2021 09:40

Physics, 01.06.2021 09:40

Mathematics, 01.06.2021 09:40

Biology, 01.06.2021 09:40


Spanish, 01.06.2021 09:40

Spanish, 01.06.2021 09:40

Spanish, 01.06.2021 09:40

Chemistry, 01.06.2021 09:40

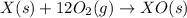
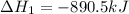 (1)
(1)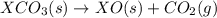
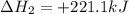 (2)
(2)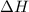 for the following reaction i.e,
for the following reaction i.e,
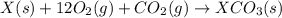
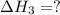 (3)
(3)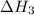 for the reaction will be:
for the reaction will be: