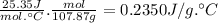Chemistry, 09.12.2019 22:31 tannerlynn7227
It takes 49.0j to raise the temperature of an 11.5g piece of unknown metal from 13.0? c to 24.3? c. what is the specific heat for the metal? express your answer numerically, in j/g? ? molar heat capacity of silver is 25.35 j/mol? ? c. how much energy would it take to raise the temperature of 11.5g of silver by 10.1? c? express your answer numerically, in is the specific heat of silver?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3
You know the right answer?
It takes 49.0j to raise the temperature of an 11.5g piece of unknown metal from 13.0? c to 24.3? c....
Questions

Spanish, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10

Biology, 18.03.2021 03:10


Physics, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10


Mathematics, 18.03.2021 03:10

Chemistry, 18.03.2021 03:10

English, 18.03.2021 03:10



History, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10




History, 18.03.2021 03:10