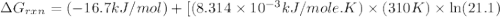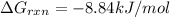Chemistry, 09.12.2019 23:31 monstergirl25
Consider the fructose-1,6-bisphosphatase reaction. calculate the free energy change if the ratio of the concentrations of the products to the concentrations of the reactants is 21.1 and the temperature is 37.0 ° c ? δ g ° ' for the reaction is − 16.7 kj/mol . δ g = kj / mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3


Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
Consider the fructose-1,6-bisphosphatase reaction. calculate the free energy change if the ratio of...
Questions







English, 18.03.2021 02:20




Mathematics, 18.03.2021 02:20


Biology, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20

Biology, 18.03.2021 02:20

Social Studies, 18.03.2021 02:20


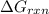 is -8.84 kJ/mol
is -8.84 kJ/mol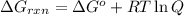 ............(1)
............(1)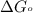 = standard Gibbs free energy = -16.7 kJ/mol
= standard Gibbs free energy = -16.7 kJ/mol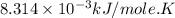
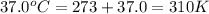
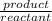 = 21.1
= 21.1