

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
Given that kp = 3.5 x 10-4 for the reaction 2 co(g) < => c(graphite) + co2(g), what is the pa...
Questions

English, 29.04.2021 09:20



History, 29.04.2021 09:20


English, 29.04.2021 09:20


Mathematics, 29.04.2021 09:20



Chemistry, 29.04.2021 09:30

Mathematics, 29.04.2021 09:30

English, 29.04.2021 09:30


History, 29.04.2021 09:30


Mathematics, 29.04.2021 09:30

Spanish, 29.04.2021 09:30



 for above reaction follows:
for above reaction follows:
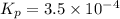
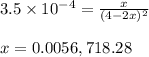
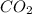 = 0.0056 atm
= 0.0056 atm

