
Chemistry, 10.12.2019 00:31 devarious83
Glycerol (c3h8o3, 92.1 g/mol) is a nonvolatile nonelectrolyte substance. consider that you have an aqueous solution that contains 32.7 % glycerol by mass. if the vapor pressure of pure water is 23.8 torr at 25oc, what is the vapor pressure of the solution at 25oc? enter your answer in units of torr to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 15:50
Astable atom that has a large nucleus most likely contains 1. more neutrons than protons. 2.more protons than neutrons. 3.equal numbers of protons and neutrons. 4.changing numbers of protons and neutrons.
Answers: 2
You know the right answer?
Glycerol (c3h8o3, 92.1 g/mol) is a nonvolatile nonelectrolyte substance. consider that you have an a...
Questions






Computers and Technology, 18.02.2020 05:34



English, 18.02.2020 05:35

Mathematics, 18.02.2020 05:35

History, 18.02.2020 05:35



Computers and Technology, 18.02.2020 05:35

Mathematics, 18.02.2020 05:35



Mathematics, 18.02.2020 05:35


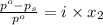
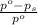 = relative lowering in vapor pressure
= relative lowering in vapor pressure
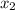 = mole fraction of solute =
= mole fraction of solute =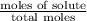
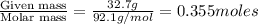
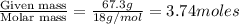
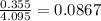
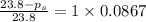
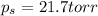
 is 21.7 torr
is 21.7 torr


