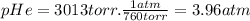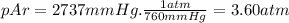Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

You know the right answer?
Amixture of he he , n 2 n2 , and ar ar has a pressure of 24.1 24.1 atm at 28.0 28.0 °c. if the parti...
Questions

Physics, 16.04.2020 02:27




Chemistry, 16.04.2020 02:27



Biology, 16.04.2020 02:27



Medicine, 16.04.2020 02:27


History, 16.04.2020 02:27


History, 16.04.2020 02:27