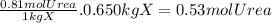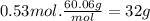Chemistry, 10.12.2019 01:31 Leanylopez0811
Acertain substance x has a normal boiling point of 124.2 °c and a molal boiling point elevation constant k,-06-0c-kg-mol ·a solution is prepared by dissolving some urea ((n112)co) in 650. g ofl. this solution boils at 124.7 oc, calculate the mass of urea that was dissolved. be sure your answer has the correct number of significant digits. 31.48 g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
How many miles of calcium oxide will be produced when 1.6 miles of iron (iii) oxide react with calcium phosphate
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3


Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Acertain substance x has a normal boiling point of 124.2 °c and a molal boiling point elevation cons...
Questions


Mathematics, 09.03.2021 22:00


Biology, 09.03.2021 22:00


Mathematics, 09.03.2021 22:00

Mathematics, 09.03.2021 22:00

Chemistry, 09.03.2021 22:00

Mathematics, 09.03.2021 22:00




Mathematics, 09.03.2021 22:00


Mathematics, 09.03.2021 22:00

Chemistry, 09.03.2021 22:00




Chemistry, 09.03.2021 22:00