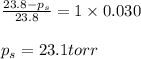Chemistry, 10.12.2019 01:31 romaguera06
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250.0 ml of water. the vapor pressure of pure water at 25°c is 23.8 torr.70.8 torr7.29 torr72.9 torr22.9 torr23.1 torr

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250....
Questions


Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

History, 20.09.2020 07:01


Biology, 20.09.2020 07:01






Mathematics, 20.09.2020 07:01


Physics, 20.09.2020 07:01


Spanish, 20.09.2020 07:01


Spanish, 20.09.2020 07:01


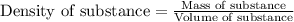
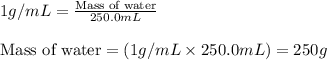
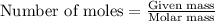 .....(1)
.....(1)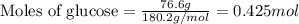
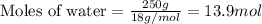
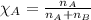
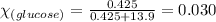
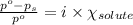
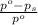 = relative lowering in vapor pressure
= relative lowering in vapor pressure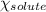 = mole fraction of solute = 0.030
= mole fraction of solute = 0.030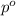 = vapor pressure of pure water = 23.8 torr
= vapor pressure of pure water = 23.8 torr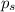 = vapor pressure of solution = ?
= vapor pressure of solution = ?