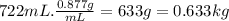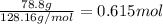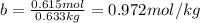Chemistry, 10.12.2019 01:31 erinwebsterrr
Determine the freezing point of a solution that contains 78.8 g of naphthalene (c10h8, molar mass = 128.16 g/mol) dissolved in 722 ml of benzene (d = 0.877 g/ml). pure benzene has a melting point of 5.50°c and a freezing point depression constant of 4.90°c/m.0.74°c4.76°c4.17°c1.68°c1. 33°c

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

You know the right answer?
Determine the freezing point of a solution that contains 78.8 g of naphthalene (c10h8, molar mass =...
Questions


Mathematics, 02.03.2021 22:10


English, 02.03.2021 22:10




Business, 02.03.2021 22:10

Biology, 02.03.2021 22:10


Mathematics, 02.03.2021 22:10



Mathematics, 02.03.2021 22:10

Mathematics, 02.03.2021 22:10


Mathematics, 02.03.2021 22:10

Mathematics, 02.03.2021 22:10


Mathematics, 02.03.2021 22:10