
Chemistry, 10.12.2019 01:31 MemnochRize
Decide if the following statements regarding intermolecular forces are true or false. 1. hf will have a higher vapor pressure at 25oc than kcl. 2. ch3ch2oh will have a higher melting point than ch3och3. 3. as the polarity of a covalent compound increases, the solubility of the compound in ccl4 decreases. 4. nh3 will have a lower vapor pressure at -50oc than no. 5. ch3ch2ch3 will have a higher boiling point than ch3ch3. explain the answers as well.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Decide if the following statements regarding intermolecular forces are true or false. 1. hf will hav...
Questions







History, 30.06.2019 05:30






Mathematics, 30.06.2019 05:30


Chemistry, 30.06.2019 05:30

Biology, 30.06.2019 05:30




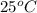 than KCl is true.
than KCl is true.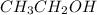 molecule, there will be hydrogen bonding which is stronger than dipole forces. Hence, it will have high melting point. Whereas in
molecule, there will be hydrogen bonding which is stronger than dipole forces. Hence, it will have high melting point. Whereas in 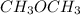 there will be dipole forces due to which there will be weak intermolecular forces leading to low melting point.
there will be dipole forces due to which there will be weak intermolecular forces leading to low melting point. 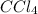 because
because 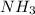 molecule there will be hydrogen bonding due to which it will have strong intermolecular forces leading to a lower vapor pressure as compared to NO in which dipole forces exist.
molecule there will be hydrogen bonding due to which it will have strong intermolecular forces leading to a lower vapor pressure as compared to NO in which dipole forces exist.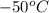 than NO, is true.
than NO, is true. 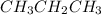 will have a higher boiling point than CH_3CH_3, is true.
will have a higher boiling point than CH_3CH_3, is true. 

