
Chemistry, 10.12.2019 01:31 twistedhyperboles
Copper-i reacts with neocuproine to form (neocuproine)2cu+, with an absorption maximum at 454nm and a molar absorbtivity of 7.90×103 m-1cm-1 when measured in isoamyl alcohol. a rock containing copper is pulverized and digested with strong acid dissolving copper and other metals. the acid is neutralized with base and the resulting solution is diluted to a total volume of 250.0 ml (solution a).10.00 ml of solution a is transferred to a new flask and treated with 10.00 ml of a reducing agent to convert all of the copper to copper-i. then 10.00 ml of buffer is added to adjust the ph (solution b).15.00 ml of solution b is withdrawn and placed in a new flask. 10.00 ml of neocuproine and 20.00 ml of isoamyl alcohol are added to the flask and it is shaken well. after settling, the isoamyl alcohol layer containing all of the copper-i complex is removed and the absorbance is measured at 454nm in a 1.0 cm cell. a blank carried through the same procedure gave an absorbance of 0.056. calculate the milligrams of copper metal in an unknown that gives an absorbance of 0.874. i recommend that you start by calculating the concentration of copper in the isoamyl alcohol layer and then perform dilution calculations until you reach the concentration in solution a.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Copper-i reacts with neocuproine to form (neocuproine)2cu+, with an absorption maximum at 454nm and...
Questions




Biology, 14.06.2021 23:40

Mathematics, 14.06.2021 23:40

English, 14.06.2021 23:40


Mathematics, 14.06.2021 23:40

History, 14.06.2021 23:40

Geography, 14.06.2021 23:40






Mathematics, 14.06.2021 23:40




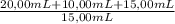 = 3,11x10⁻⁴M Copper
= 3,11x10⁻⁴M Copper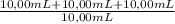 = 9,32x10⁻⁴M Copper
= 9,32x10⁻⁴M Copper = 0,0148 g of Copper ≡ 14,8 mg of Copper in an unknown
= 0,0148 g of Copper ≡ 14,8 mg of Copper in an unknown

