Chemistry, 10.12.2019 01:31 quiyansimmons15
At a given temperature the vapor pressures of benzene and toluene are 183 mm hg and 59.2 mm hg, respectively. calculate the total vapor pressure over a solution of benzene and toluene with xbenzene = 0.580.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

You know the right answer?
At a given temperature the vapor pressures of benzene and toluene are 183 mm hg and 59.2 mm hg, resp...
Questions

Mathematics, 15.07.2020 20:01




Mathematics, 15.07.2020 20:01



Law, 15.07.2020 20:01





History, 15.07.2020 20:01







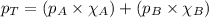
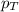 = total vapor pressure
= total vapor pressure