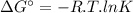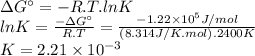Chemistry, 10.12.2019 01:31 mariahdelossantos031
The free energy change δgt at 2400 k is equal to 1.22 x 10^5j/mol. calculate the equilibrium constant at 2400 k. express your answer numerically.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

You know the right answer?
The free energy change δgt at 2400 k is equal to 1.22 x 10^5j/mol. calculate the equilibrium constan...
Questions



English, 24.06.2020 15:01


Mathematics, 24.06.2020 15:01


Mathematics, 24.06.2020 15:01


Mathematics, 24.06.2020 15:01


Mathematics, 24.06.2020 15:01


Mathematics, 24.06.2020 15:01



Mathematics, 24.06.2020 15:01

Chemistry, 24.06.2020 15:01

Mathematics, 24.06.2020 15:01


Social Studies, 24.06.2020 15:01