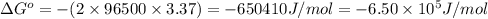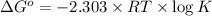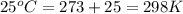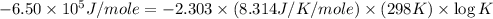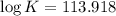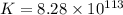Chemistry, 10.12.2019 01:31 adajadavis2843
Consider the following reaction. mg(s) + 2vo2+ (aq)+ 4 h+(aq) → mg2+(aq) + 2 vo2+(aq) + h2o(l)e⁰cell = 3.37 v. a.calculate the δg⁰∘ for the reaction. express your answer to three significant figures. b. calculate the k for the reaction. express your answer to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Consider the following reaction. mg(s) + 2vo2+ (aq)+ 4 h+(aq) → mg2+(aq) + 2 vo2+(aq) + h2o(l)e⁰cell...
Questions





Computers and Technology, 01.05.2021 22:00


Mathematics, 01.05.2021 22:10

Mathematics, 01.05.2021 22:10


Mathematics, 01.05.2021 22:10

English, 01.05.2021 22:10

Mathematics, 01.05.2021 22:10

Mathematics, 01.05.2021 22:10

Spanish, 01.05.2021 22:10



World Languages, 01.05.2021 22:10

Mathematics, 01.05.2021 22:10


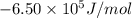
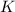 at 298 is,
at 298 is, 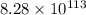
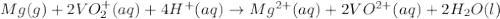
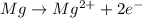
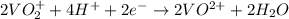
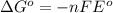
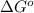 = Gibbs free energy = ?
= Gibbs free energy = ?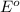 = standard e.m.f of cell = 3.37 V
= standard e.m.f of cell = 3.37 V