
Chemistry, 10.12.2019 01:31 bonetanne7171
Consider a solution containing 3.54 mm of an analyte, x, and 1.23 mm of a standard, s. upon chromatographic separation of the solution peak areas for x and s are 3275 and 10829, respectively. determine the response factor for x relative to s. f=to determine the concentration of x in an unknown solution, 1.00 ml of 8.27 mm s was added to 5.00 ml of the unknown x solution and the mixture was diluted to 10.0 ml. after chromatographic separation, this solution gave peak areas of 5389 and 4627 for x and s, respectively. determine the concentration of s in the 10.0 ml solution.[s]=determine the concentration of x in the 10.0 ml solution.[x]=determine the concentration of x in the unknown solution.[x]=

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Consider a solution containing 3.54 mm of an analyte, x, and 1.23 mm of a standard, s. upon chromato...
Questions





Mathematics, 15.01.2021 23:00



Mathematics, 15.01.2021 23:00

English, 15.01.2021 23:00



Mathematics, 15.01.2021 23:00

Spanish, 15.01.2021 23:00

Mathematics, 15.01.2021 23:00

Physics, 15.01.2021 23:00

English, 15.01.2021 23:00

Mathematics, 15.01.2021 23:00


Spanish, 15.01.2021 23:00

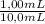 = 0,827mM
= 0,827mM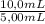 = 18,3mM
= 18,3mM

