
Chemistry, 10.12.2019 02:31 Kimberlytdb
Calculate the change in enthalpy for the following reaction, using the bond enthalpy data provided below. (note: you may round the bond energies to the nearest 100 kj/mol during your calculations.) 2 n2h2(g) 3 o2(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Calculate the change in enthalpy for the following reaction, using the bond enthalpy data provided b...
Questions




Mathematics, 05.04.2021 21:50


English, 05.04.2021 21:50

Mathematics, 05.04.2021 21:50


Mathematics, 05.04.2021 21:50

Physics, 05.04.2021 21:50



Mathematics, 05.04.2021 21:50

Mathematics, 05.04.2021 21:50


Mathematics, 05.04.2021 21:50


Mathematics, 05.04.2021 21:50


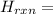 [2(409) + 4(388) + 3(496) - 4(630) - 4(463)] kJ/mol = 818 + 1552 + 1488 - 2520 - 1852 = -514 kJ/mol
[2(409) + 4(388) + 3(496) - 4(630) - 4(463)] kJ/mol = 818 + 1552 + 1488 - 2520 - 1852 = -514 kJ/mol

