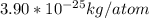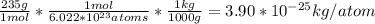Find the mass of one atom of uranium-235. recall that the mass in atomic mass units is equal to the mass in grams of one mole of atoms. avagadro's number, 6.022×1023atoms/mole, gives the number of atoms in one mole. give your answer in kilograms to three significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Find the mass of one atom of uranium-235. recall that the mass in atomic mass units is equal to the...
Questions



Mathematics, 23.06.2019 03:40


Mathematics, 23.06.2019 03:40

Mathematics, 23.06.2019 03:40

Chemistry, 23.06.2019 03:40

Mathematics, 23.06.2019 03:40

English, 23.06.2019 03:40


English, 23.06.2019 03:40

Mathematics, 23.06.2019 03:40

Mathematics, 23.06.2019 03:40




Mathematics, 23.06.2019 03:40



History, 23.06.2019 03:40