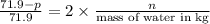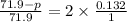Asolution of sodium chloride in water has a vapor pressure of 17.5 torr at 25°c. what is the mole fraction of nacl solute particles in this solution? what would be the vapor pressure of this solution at 45°c? the vapor pressure of pure water is 23.8 torr at 25°c and 71.9 torr at 45°c and assume sodium chloride exists as na⁺ and cl⁻ ions in solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3

Chemistry, 23.06.2019 13:00
How many grams of oxygen gas will react completely with a block of calcium metal that is 3.0 cm by 3.5 cm by 4.2 cm, if the density of calcium is 1.55 g/ml? show all steps of your calculation as well as the final answer.
Answers: 3
You know the right answer?
Asolution of sodium chloride in water has a vapor pressure of 17.5 torr at 25°c. what is the mole fr...
Questions







Mathematics, 31.07.2020 21:01






Social Studies, 31.07.2020 21:01





Physics, 31.07.2020 21:01


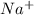 and
and 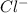 in solution. Therefore, Van't Hoff factor (i) will be equal to 2.
in solution. Therefore, Van't Hoff factor (i) will be equal to 2.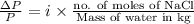
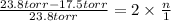
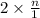
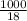
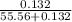
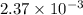
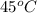 , the vapor pressure will be calculated as follows.
, the vapor pressure will be calculated as follows.