
Chemistry, 10.12.2019 02:31 Maelynne8515
Consider the reaction, cl2 + h2s => 2 hcl + s, which is found to be first order in cl2. which step of the proposed mechanism must be slow in order to agree with this rate law? cl2 => 2 cl cl + h2s => hcl + hs cl + hs => hcl + s
a. only 3
b. only 2
c. 1
d. either 2 or 3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

You know the right answer?
Consider the reaction, cl2 + h2s => 2 hcl + s, which is found to be first order in cl2. which st...
Questions

Spanish, 18.12.2020 22:00

Mathematics, 18.12.2020 22:00

Mathematics, 18.12.2020 22:00



Business, 18.12.2020 22:00

Mathematics, 18.12.2020 22:00



English, 18.12.2020 22:00


English, 18.12.2020 22:00

Health, 18.12.2020 22:00



History, 18.12.2020 22:00


Engineering, 18.12.2020 22:00


Mathematics, 18.12.2020 22:00

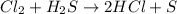
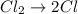
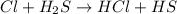
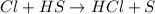
![rate=k[Cl_{2}]^\frac{1}{2}[H_{2}S]](/tpl/images/0410/9890/7d770.png)


