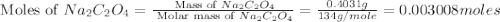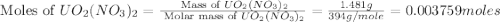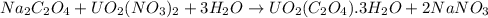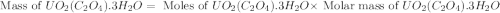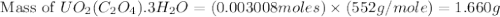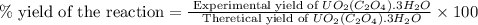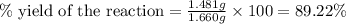Chemistry, 10.12.2019 04:31 hemolelekeakua
Uranium can be isolated from its ores by dissolving it as uo2(no3)2, then separating it as solid uo2(c2o4)*3h2o. addition of 0.4031 g of sodium oxalate, na2c2o4, to a solution containing 1.481 g of uranyl nitrate, uo2(no3)2, yields 1.073 g of solid uo2(c2o4)*3h2o.
na2c2o4 + uo2(no3)2 + 3h2o > uo2(c2o4)*3h2o + 2nano3
1. determine the limiting reactant and the percent yield of this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1



Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
Uranium can be isolated from its ores by dissolving it as uo2(no3)2, then separating it as solid uo2...
Questions




Mathematics, 24.05.2021 22:30

Chemistry, 24.05.2021 22:30



Mathematics, 24.05.2021 22:30


Chemistry, 24.05.2021 22:30

Arts, 24.05.2021 22:30

Arts, 24.05.2021 22:30




Mathematics, 24.05.2021 22:30

Mathematics, 24.05.2021 22:30

Geography, 24.05.2021 22:30

History, 24.05.2021 22:30

Computers and Technology, 24.05.2021 22:30

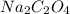
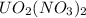 = 1.481 g
= 1.481 g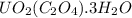 = 552 g/mole
= 552 g/mole