
Chemistry, 10.12.2019 04:31 sbudlove2838
Gfind the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h8(g) + 5o2(g) → 3co2(g) + 4h2o(g) ∆h = −2043 kj c(s) + o2(g) → co2(g) ∆h = −393.5 kj 2h2(g) + o2(g) → 2h2o(g) ∆h = −483.6 kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the value of ksp for caf2 at 25°c is 4.0 x 10-11. will this solution form a precipitate? yes no
Answers: 3

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1
You know the right answer?
Gfind the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h...
Questions

Biology, 11.06.2021 04:10



English, 11.06.2021 04:10


English, 11.06.2021 04:10

Mathematics, 11.06.2021 04:10

English, 11.06.2021 04:10


Mathematics, 11.06.2021 04:10

Mathematics, 11.06.2021 04:10




Mathematics, 11.06.2021 04:10



Mathematics, 11.06.2021 04:10

English, 11.06.2021 04:10

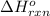 for the reaction is -104.7 kJ.
for the reaction is -104.7 kJ.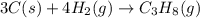
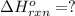
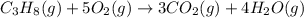
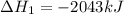
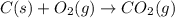
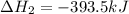 ( × 3)
( × 3) 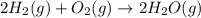
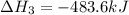 ( × 2)
( × 2) ![\Delta H^o_{rxn}=[1\times (-\Delta H_1)]+[3\times \Delta H_2]+[2\times \Delta H_3]](/tpl/images/0411/2485/b4dbe.png)
![\Delta H^o_{rxn}=[(1\times (-(-2043))+(3\times (-393.5))+(2\times (-483.6))]=-104.7kJ](/tpl/images/0411/2485/eafbb.png)


