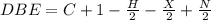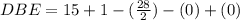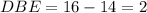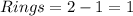Chemistry, 10.12.2019 04:31 areeves39276
Anewly isolated natural product was found to have the molecular formula c15h28o2. by hydrogenating a sample of the compound, it was determined to possess one pi bond. how many rings are present in the compound?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1


Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2
You know the right answer?
Anewly isolated natural product was found to have the molecular formula c15h28o2. by hydrogenating a...
Questions


History, 10.11.2019 07:31

Mathematics, 10.11.2019 07:31



Mathematics, 10.11.2019 07:31



English, 10.11.2019 08:31


Mathematics, 10.11.2019 08:31


Advanced Placement (AP), 10.11.2019 08:31

Mathematics, 10.11.2019 08:31



Mathematics, 10.11.2019 08:31


World Languages, 10.11.2019 08:31