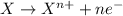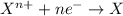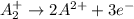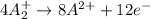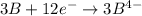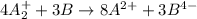Chemistry, 10.12.2019 04:31 summerhumphries3
Given two half reactions as follows: a2+ → 2 a2+ + 3 e− 4 e− + b → b4− what would you multiply each half-reaction by, to cancel out the electrons?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
Given two half reactions as follows: a2+ → 2 a2+ + 3 e− 4 e− + b → b4− what would you multiply each...
Questions


Physics, 02.10.2020 14:01




Biology, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01


History, 02.10.2020 14:01




Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01