
Chemistry, 10.12.2019 04:31 monsterwins5001
Ammonia will decompose into nitrogen and hydrogen at high temperature. an industrial chemist studying this reaction fills a 500. ml flask with 2.3 atm of ammonia gas at 32. °c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 0.69 atm. calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. an industrial chemist studyin...
Questions


Mathematics, 27.07.2019 09:00

Social Studies, 27.07.2019 09:00

Mathematics, 27.07.2019 09:00


History, 27.07.2019 09:00


Mathematics, 27.07.2019 09:00


History, 27.07.2019 09:00

Business, 27.07.2019 09:00



Mathematics, 27.07.2019 09:00



Biology, 27.07.2019 09:00

English, 27.07.2019 09:00


History, 27.07.2019 09:00

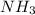 is as follows:.
is as follows:.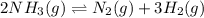
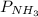 = 2.3 atm at equilibrium
= 2.3 atm at equilibrium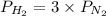 = 0.69 atm
= 0.69 atm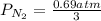
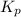 will be as follows.
will be as follows.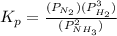
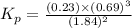
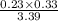
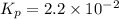
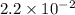 .
.

