Chemistry, 10.12.2019 05:31 star030616
Asample of calcium carbonate (caco3) is known to contain some impurities. it is found that calcium makes up 11% of the entire mass of the sample. all of the calcium comes from the caco3 compound. find the mass percent of caco3 in the sample. set up a ratio and solve.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1


You know the right answer?
Asample of calcium carbonate (caco3) is known to contain some impurities. it is found that calcium m...
Questions

Biology, 28.04.2021 20:50

Biology, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50




Mathematics, 28.04.2021 20:50


English, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50

Mathematics, 28.04.2021 20:50


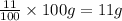
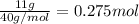
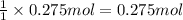 calcium carbonate
calcium carbonate