
Chemistry, 10.12.2019 05:31 annabanana1298
1‑propanol ( n ‑propanol) and 2‑propanol (isopropanol) form ideal solutions in all proportions. calculate the partial pressure and the mole fraction ( y ) of the vapor phase of each component in equilibrium with each of the given solutions at 25 °c. p ∘ prop = 20.9 torr and p ∘ iso = 45.2 torr at 25 °c. a solution with a mole fraction of x prop = 0.247 .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2


Chemistry, 23.06.2019 12:30
D5w is shorthand for a 5% glucose rehydration fluid used in ivs. the doctor orders 1200 ml d5w@ 30 gtts/min. you have an iv tube that delivers 18 gtts/cc. how many hours will it take for the 1200 cc bottle to infuse?
Answers: 2
You know the right answer?
1‑propanol ( n ‑propanol) and 2‑propanol (isopropanol) form ideal solutions in all proportions. calc...
Questions

Chemistry, 13.07.2019 05:00


Chemistry, 13.07.2019 05:00

Advanced Placement (AP), 13.07.2019 05:00



History, 13.07.2019 05:00

English, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00


History, 13.07.2019 05:00



English, 13.07.2019 05:00




Social Studies, 13.07.2019 05:00


Biology, 13.07.2019 05:00

 = 0.134;
= 0.134;  = 0.866
= 0.866 ) = 1 - mole fraction of propanol (
) = 1 - mole fraction of propanol (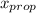 ).
). 
 is the partial pressure of propanol and
is the partial pressure of propanol and 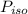 is the partial pressure of isopropanol.
is the partial pressure of isopropanol.

