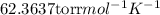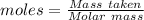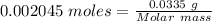Chemistry, 10.12.2019 06:31 Alexandragurule18
(a) the mass density of a gaseous compound was found to be 1.23 kg m^−3 at 330 k and 20 kpa. what is the molar mass of the compound? (b) in an experiment to measure the molar mass of a gas, 250 cm3 of the gas was confined in a glass vessel. the pressure was 152 torr at 298 k, and after correcting for buoyancy effects, the mass of the gas was 33.5 mg. what is the molar mass of the gas?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 15:30
The gas in a sealed container has an absolute pressure of 9.25 atmospheres. if the air around the container is at standard pressure, what is the gauge pressure inside the container
Answers: 1

Chemistry, 23.06.2019 18:00
Explain how compaction is important in the formation of coal.
Answers: 1
You know the right answer?
(a) the mass density of a gaseous compound was found to be 1.23 kg m^−3 at 330 k and 20 kpa. what is...
Questions

Social Studies, 03.07.2019 16:30

Social Studies, 03.07.2019 16:30


Business, 03.07.2019 16:30


History, 03.07.2019 16:30

History, 03.07.2019 16:30

History, 03.07.2019 16:30


History, 03.07.2019 16:30

Social Studies, 03.07.2019 16:30



Mathematics, 03.07.2019 16:30


Social Studies, 03.07.2019 16:30


Mathematics, 03.07.2019 16:30

Chemistry, 03.07.2019 16:30

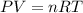
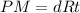
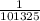 P (atm)
P (atm)
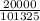 atm
atm