Chemistry, 10.12.2019 06:31 murphyscott794
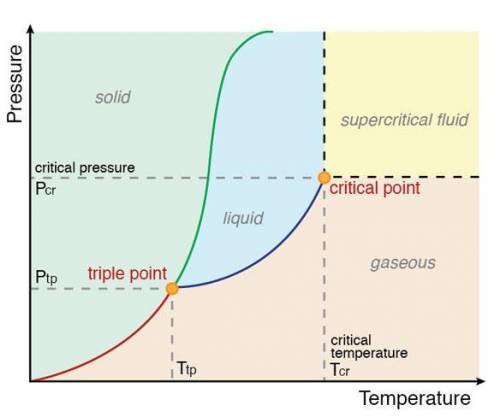
Acertain substance, x, has a triple-point temperature of 20°c at a pressure of 2.0 atm.
which one of the following statements cannot possibly be true?
a) x can exist as a liquid above 20°c.
b) x can exist as a solid above 20°c.
c) liquid x can exist as a stable phase at 25°c, 1 atm.
d) both liquid and solid x have the same vapor pressure at 20°c.
e) all of these statements could be true

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
Acertain substance, x, has a triple-point temperature of 20°c at a pressure of 2.0 atm.
...
...
Questions


Mathematics, 27.12.2019 00:31

Mathematics, 27.12.2019 00:31

Health, 27.12.2019 00:31

Mathematics, 27.12.2019 00:31




Biology, 27.12.2019 00:31



Biology, 27.12.2019 00:31


Mathematics, 27.12.2019 00:31