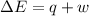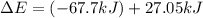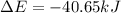Chemistry, 10.12.2019 06:31 wendelkristen
Consider a balloon filled with helium at the following conditions. 313 g he 1.00 atm 1910. l molar heat capacity = 20.8 j/degree c middot mol the temperature of this balloon is decreased by 41.6 degree c as the volume decreases to 1643 l with the pressure remaining constant. determine q, w, and delta e (in kj) for the compression of the balloon.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 17:50
How do chemical equations demonstrate conservation of mass
Answers: 1
You know the right answer?
Consider a balloon filled with helium at the following conditions. 313 g he 1.00 atm 1910. l molar h...
Questions

Mathematics, 09.03.2021 19:00



Mathematics, 09.03.2021 19:00

Biology, 09.03.2021 19:00

Geography, 09.03.2021 19:00



Chemistry, 09.03.2021 19:00

English, 09.03.2021 19:00


Mathematics, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00


Mathematics, 09.03.2021 19:00



English, 09.03.2021 19:00


English, 09.03.2021 19:00

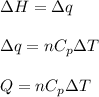
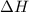 = change in enthalpy energy
= change in enthalpy energy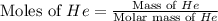
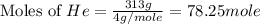
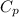 = heat capacity at constant pressure =
= heat capacity at constant pressure = 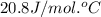
 = change in temperature =
= change in temperature = 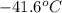
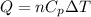
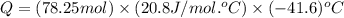
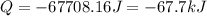
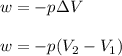
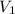 = initial volume = 1910 L
= initial volume = 1910 L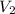 = final volume = 1643 L
= final volume = 1643 L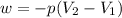
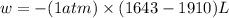
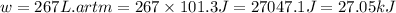
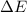 of the gas.
of the gas.