
Chemistry, 10.12.2019 07:31 CobyHageman
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, hcl ( aq ) , as described by the chemical equation
mno 2 ( s ) + 4 hcl ( aq ) ⟶ mncl 2 ( aq ) + 2 h 2 o ( l ) + cl 2 ( g )
how much mno 2 ( s ) should be added to excess hcl ( aq ) to obtain 255 ml cl 2 ( g ) at 25 °c and 795 torr ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions











Computers and Technology, 02.07.2021 01:00




Mathematics, 02.07.2021 01:00

English, 02.07.2021 01:00

English, 02.07.2021 01:00



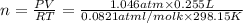
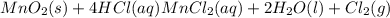
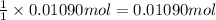 manganese dioxide
manganese dioxide

