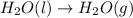Chemistry, 22.08.2019 16:40 sadieismichaeljackso
Regarding the vaporization of water, which of the following is true? a. a great deal of heat must be lost by water to form hydrogen bonds and allow molecules to move farther apart. b. a great deal of heat must be absorbed by water to break hydrogen bonds and allow molecules to move farther apart. c. vaporization of water is an exothermic process. d. water must lose a great deal of heat before it will vaporize.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
Regarding the vaporization of water, which of the following is true? a. a great deal of heat must b...
Questions

History, 19.07.2019 07:30

Biology, 19.07.2019 07:30



Business, 19.07.2019 07:30


History, 19.07.2019 07:30




Biology, 19.07.2019 07:30

History, 19.07.2019 07:30





Mathematics, 19.07.2019 07:30


Mathematics, 19.07.2019 07:30

Mathematics, 19.07.2019 07:30