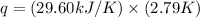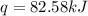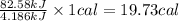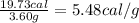Aresearcher studying the nutritional value of a new candy places a 3.60 g sample of the candy inside a bomb calorimeter and combusts it in excess oxygen. the observed temperature increase is 2.79 ∘ c. if the heat capacity of the calorimeter is 29.60 kj ⋅ k − 1 , how many nutritional calories are there per gram of the candy?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
Aresearcher studying the nutritional value of a new candy places a 3.60 g sample of the candy inside...
Questions


History, 07.12.2020 18:10


Social Studies, 07.12.2020 18:10

Physics, 07.12.2020 18:10


Health, 07.12.2020 18:10

Mathematics, 07.12.2020 18:10

History, 07.12.2020 18:10

Engineering, 07.12.2020 18:10

Mathematics, 07.12.2020 18:10

Physics, 07.12.2020 18:10




History, 07.12.2020 18:10

Computers and Technology, 07.12.2020 18:10


Mathematics, 07.12.2020 18:10

History, 07.12.2020 18:10

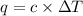
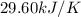
 = change in temperature =
= change in temperature = 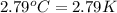 (Change in kelvin temperature = Change in Celsius temperature)
(Change in kelvin temperature = Change in Celsius temperature)