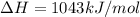Calculate the enthalpy of reaction for 2co + o2 → 2co2. given the following bond energies:
b...

Chemistry, 10.12.2019 19:31 cdjeter12oxoait
Calculate the enthalpy of reaction for 2co + o2 → 2co2. given the following bond energies:
be(c≡o) = 1074 kj/mol
be(o≡o) = 499 kj/mol
be(c≡o) = 802 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2


Chemistry, 23.06.2019 20:00
Assume you inhale in about 6 l of air per minute how much nitrogen do you breathe in one minute
Answers: 1
You know the right answer?
Questions



Mathematics, 19.08.2019 16:20



Geography, 19.08.2019 16:20

Mathematics, 19.08.2019 16:20


Social Studies, 19.08.2019 16:20

English, 19.08.2019 16:20





Mathematics, 19.08.2019 16:20


History, 19.08.2019 16:20


Mathematics, 19.08.2019 16:20

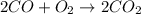
![\Delta H=[(2\times B.E_{C\equiv O})+(1\times B.E_{O\equiv O})]-[2\times B.E_{C=O}]](/tpl/images/0412/0179/8905d.png)
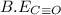 = 1074 kJ/mol
= 1074 kJ/mol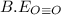 = 499 kJ/mol
= 499 kJ/mol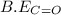 = 802 kJ/mol
= 802 kJ/mol![\Delta H=[(2\times 1074kJ/mol)+(1\times 499kJ/mol)]-[2\times 802kJ/mol]](/tpl/images/0412/0179/c40d4.png)