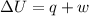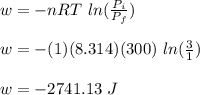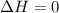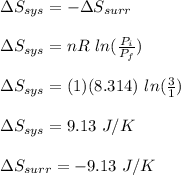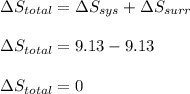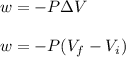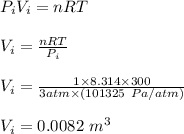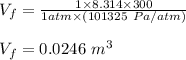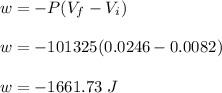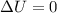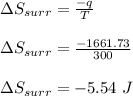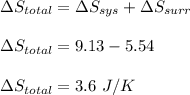Asample consisting of 1.00 mol of perfect gas molecules at 27 °c is expanded isothermally from an initial pressure of 3.00 atm to a final pressure of 1.00 atm in two ways: (a) reversibly, and (b) against a constant external pressure of 1.00 atm. evaluate q, w, δu, δh, δs, δssurr, and δstot in each case.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Asample consisting of 1.00 mol of perfect gas molecules at 27 °c is expanded isothermally from an in...
Questions



Mathematics, 30.06.2021 22:10

English, 30.06.2021 22:10

Mathematics, 30.06.2021 22:10


Mathematics, 30.06.2021 22:10

History, 30.06.2021 22:10

Mathematics, 30.06.2021 22:10


Mathematics, 30.06.2021 22:10

Mathematics, 30.06.2021 22:10








Mathematics, 30.06.2021 22:10

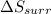 is -9.13 J/K, the entropy change of the system,
is -9.13 J/K, the entropy change of the system, 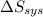 is 9.13 J/K, and the total entropy change,
is 9.13 J/K, and the total entropy change, 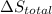 is 0.
is 0.