
Chemistry, 10.12.2019 21:31 keasiabradley
Water's unique properties, including its high heat capacity, high density, high boiling point and a solid phase that is less dense than its liquid phase, can best be attributed to:
a) its formula, h2o.
b) the covalent oxygen-hydrogen bonds in the molecule.
c) the shape of the molecule.
d) the polarity of the molecule and hydrogen bonding between the molecules.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1


Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
Water's unique properties, including its high heat capacity, high density, high boiling point and a...
Questions

Spanish, 05.07.2019 18:00

Mathematics, 05.07.2019 18:00


History, 05.07.2019 18:00


Mathematics, 05.07.2019 18:00


Mathematics, 05.07.2019 18:00

History, 05.07.2019 18:00

Biology, 05.07.2019 18:00

Geography, 05.07.2019 18:00


Biology, 05.07.2019 18:00


Chemistry, 05.07.2019 18:00


Mathematics, 05.07.2019 18:00

Mathematics, 05.07.2019 18:00

Mathematics, 05.07.2019 18:00

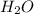 and due to the difference in electronegativity of hydrogen and oxygen atom there is formation of partial positive charge on hydrogen and partial negative charge on oxygen atom.
and due to the difference in electronegativity of hydrogen and oxygen atom there is formation of partial positive charge on hydrogen and partial negative charge on oxygen atom.

