Chemistry, 10.12.2019 22:31 Kimberlytdb
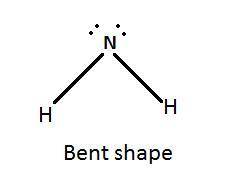
The shape (or geometry) of the amide anion, nh2-, the number of lone pairs on nitrogen, and the hybridization at nitrogen are, respectively,
a. linear, 2, and sp
b. bent, 2, and sp3
c. tetrahedral, 2, and sp3
d. bent, 1, and sp2
e. linear, 1, and sp
f. none of these answers is correct

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
The shape (or geometry) of the amide anion, nh2-, the number of lone pairs on nitrogen, and the hybr...
Questions


Mathematics, 15.12.2020 02:30

Mathematics, 15.12.2020 02:30

English, 15.12.2020 02:30

History, 15.12.2020 02:30


History, 15.12.2020 02:30





History, 15.12.2020 02:30

English, 15.12.2020 02:30

History, 15.12.2020 02:30

Mathematics, 15.12.2020 02:30

Mathematics, 15.12.2020 02:30

Mathematics, 15.12.2020 02:30


Mathematics, 15.12.2020 02:30

Mathematics, 15.12.2020 02:30

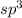
![\text{Number of electron pair}=\frac{1}{2}[V+N-C+A]](/tpl/images/0412/3834/9e987.png)
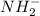
![\text{Number of electrons}=\frac{1}{2}\times [5+2+1]=4](/tpl/images/0412/3834/7ea4a.png)