
Chemistry, 11.12.2019 01:31 boogiedownclown
What is the identity of the element, and how many neutrons does it have? an elements most stable ion forms an ionic compound with chlorine having the formula xcl2. if the ion of element x has a mass of 34 and 18 electrons. a. ar, 16 neutronsb. ca, 14 neutronsc. ar, 18 neutronsd. s, 18 neutronse. k, 20 neutrons

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
What is the identity of the element, and how many neutrons does it have? an elements most stable io...
Questions


Biology, 28.06.2019 11:30



Social Studies, 28.06.2019 11:30



Biology, 28.06.2019 11:30

Mathematics, 28.06.2019 11:30


Mathematics, 28.06.2019 11:30




Health, 28.06.2019 11:30



History, 28.06.2019 11:30

Mathematics, 28.06.2019 11:30

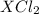 , it can be broken down into
, it can be broken down into 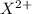 and
and 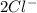 . Its ion has a mass of 34 and 18 electrons which means it has already lost 2 electrons.
. Its ion has a mass of 34 and 18 electrons which means it has already lost 2 electrons. 

